PARP Inhibitors Market Share & Growth Analysis, 2032 | UnivDatos
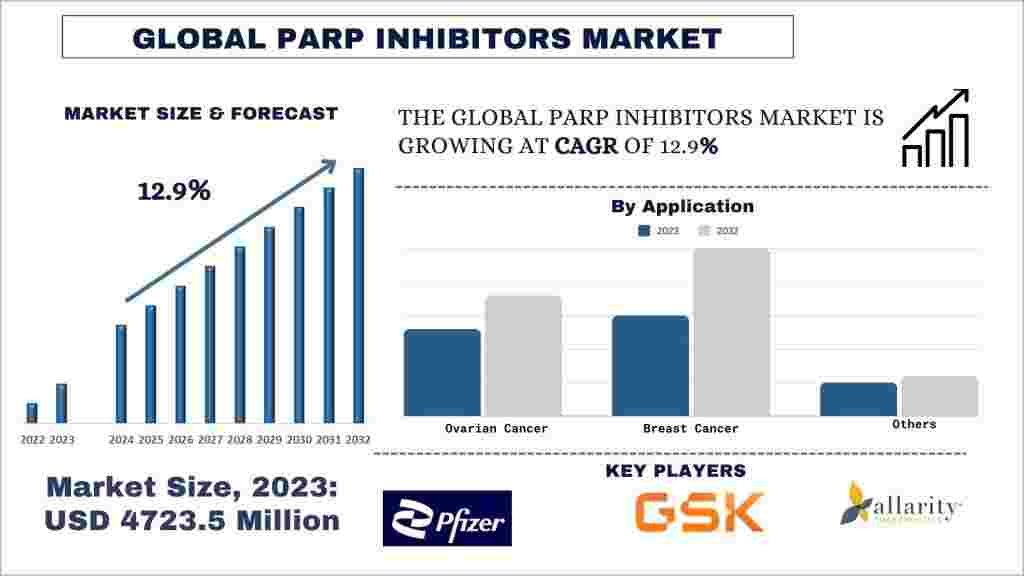
According to UnivDatos, the PARP inhibitors market was valued at USD 4,723.5 million in 2023 and is projected to grow at a robust CAGR of approximately 12.9% during the forecast period (2024–2032), driven largely by rising investments in PARP inhibitor research and development. Poly (ADP-ribose) polymerase (PARP) inhibitors have emerged as a breakthrough class of oncology therapeutics, particularly for the treatment of ovarian, breast, and prostate cancers. Over the past few years, demand for PARP inhibitors has increased significantly due to their unique mechanism of action, which targets cancer cells with defective DNA repair pathways.
This article explores the accelerating pace of clinical trials, combination therapy strategies, strategic investments, and recent product launches shaping the global PARP inhibitors landscape.
Understanding the Mechanism of PARP Inhibitors
PARP inhibitors function by blocking the PARP enzyme, which plays a critical role in repairing single-strand DNA breaks. When PARP activity is inhibited, these single-strand breaks accumulate and are converted into double-strand breaks during DNA replication. Cancer cells—especially those with deficiencies in homologous recombination repair mechanisms such as BRCA1 and BRCA2 mutations—are unable to repair this damage, leading to selective cancer cell death. This concept of synthetic lethality underpins the clinical success of PARP inhibitors in genetically defined cancer populations.
Access sample report (including graphs, charts, and figures): https://univdatos.com/reports/parp-inhibitors-market?popup=report-enquiry
Surge in Clinical Trial Activity
The number of clinical trials evaluating PARP inhibitors has expanded rapidly in recent years, reflecting growing confidence in their therapeutic potential. A key driver of this expansion is the exploration of PARP inhibitors in combination with other treatment modalities, including immunotherapies and targeted agents, to enhance efficacy and overcome resistance.
Recent Company-Led Clinical Trials
AstraZeneca and Merck (January 2024)
- Trial: Phase III PAOLA-1
- Combination: Olaparib (Lynparza) + Bevacizumab
- Indication: Advanced ovarian cancer
- Outcome: Demonstrated a significant improvement in progression-free survival, particularly in patients with HRD-positive tumors.
GlaxoSmithKline (March 2024)
- Trial: Phase II MOONSTONE
- Combination: Niraparib (Zejula) + Pembrolizumab
- Indication: Platinum-resistant ovarian cancer
- Outcome: Positive efficacy results compared to monotherapy, supporting advancement to Phase III studies.
Clovis Oncology (April 2024)
- Trial: Phase II TRITON2
- Combination: Rucaparib (Rubraca) + Enzalutamide
- Indication: Metastatic castration-resistant prostate cancer (mCRPC)
- Outcome: Showed encouraging activity in patients with BRCA mutations.
Growing Importance of Combination Therapies
Combination therapy has become a central strategy in PARP inhibitor development. By pairing PARP inhibitors with chemotherapy, immunotherapy, or hormone therapies, developers aim to improve treatment response, delay resistance, and broaden patient eligibility.
Key combinations under investigation include:
- Olaparib + Bevacizumab for advanced ovarian cancer, showing notable improvements in progression-free survival.
- Niraparib + Pembrolizumab for platinum-resistant ovarian cancer, leveraging immune checkpoint inhibition to enhance antitumor activity.
- Rucaparib + Enzalutamide for mCRPC, combining androgen receptor inhibition with DNA repair suppression.
Strategic Investments and Industry Collaborations
The surge in clinical activity has been accompanied by substantial financial investments from pharmaceutical companies and venture capital firms.
Recent Strategic Developments
- Pfizer–Myriad Genetics Collaboration (February 2024):
- A USD 500 million investment to co-develop and commercialize next-generation PARP inhibitors, focusing on expanded indications and improved delivery technologies.
- AstraZeneca Expansion (May 2024):
- AstraZeneca announced an additional USD 300 million investment to advance novel PARP inhibitor combinations and next-generation molecules.
- Clovis Oncology Funding Round (April 2024):
- Raised USD 200 million to accelerate development of its PARP inhibitor combination pipeline.
Click here to view the Report Description & TOC: https://univdatos.com/reports/parp-inhibitors-market
Recent Product Launches
The commercial landscape for PARP inhibitors continues to expand, supported by regulatory approvals and new indications.
- Lynparza (Olaparib) – AstraZeneca & Merck (January 2024):
- Approved as a first-line maintenance therapy for BRCA-mutated advanced ovarian cancer, based on strong PAOLA-1 trial outcomes.
- Zejula (Niraparib) – GlaxoSmithKline (March 2024):
- Launched for platinum-resistant ovarian cancer in combination with pembrolizumab, following accelerated approval.
- Rubraca (Rucaparib) – Clovis Oncology (April 2024):
- Introduced for BRCA-mutant mCRPC, supported by positive TRITON2 trial results.
Future Outlook of the PARP Inhibitors Market
The outlook for PARP inhibitors remains highly optimistic, driven by expanding research horizons and precision oncology advances.
Expanding Indications
Ongoing trials are evaluating PARP inhibitors in pancreatic, lung, and other solid tumors, potentially extending their use beyond BRCA-mutant cancers.
Addressing Drug Resistance
Resistance remains a key challenge, prompting research into resistance mechanisms and the development of rational combination strategies, particularly with immune checkpoint inhibitors.
Personalized Oncology
Advances in genomic profiling and biomarker-driven treatment selection are positioning PARP inhibitors at the forefront of personalized cancer therapy, ensuring better patient stratification and outcomes.
Conclusion
The rapid rise in clinical trials, combination therapies, strategic investments, and product launches underscores the growing importance of PARP inhibitors in modern oncology. With continued innovation and expanding clinical applications, PARP inhibitors are poised to play a transformative role in cancer treatment. As research advances toward overcoming resistance and broadening indications, patients stand to benefit from more targeted, effective, and personalized therapeutic options in the years ahead.
Contact Us:
UnivDatos
Contact Number - +1 978 733 0253
Email - contact@univdatos.com
Website - www.univdatos.com
Linkedin- https://www.linkedin.com/company/univ-datos-market-insight/mycompany/
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Παιχνίδια
- Gardening
- Health
- Κεντρική Σελίδα
- Literature
- Music
- Networking
- άλλο
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness



