Aligning Syringe Infusion Pump Tender Needs
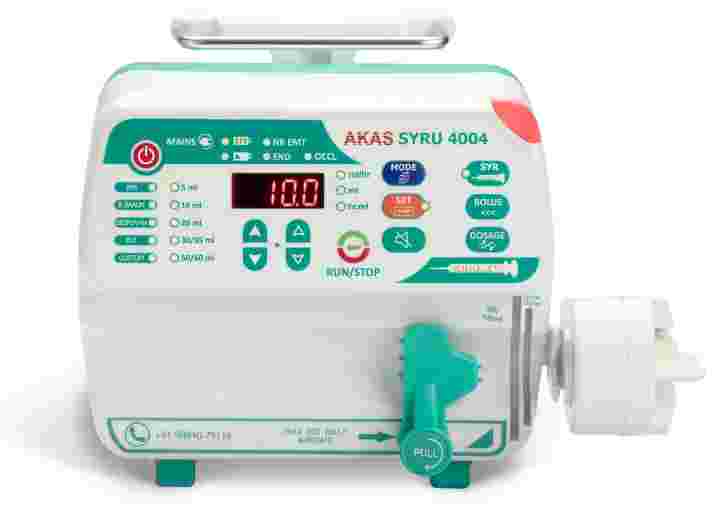
Introduction
Hospital tender processes are built around patient safety, clinical precision, and long-term operational sustainability.
Every specification included in a bid document reflects the responsibility hospitals carry toward patients and caregivers.
Selecting the right Syringe Infusion Pump therefore becomes a matter of both technical alignment and ethical commitment.
A well-structured tender ensures transparency, accountability, and measurable performance standards.
Manufacturers must understand that tenders are not simply procurement checklists but frameworks for safe drug delivery.
Aligning Syringe Infusion Pump features with these requirements protects patient outcomes and institutional credibility.
In critical care, neonatal units, oncology departments, and operating theatres, infusion accuracy directly impacts lives.
Hospitals expect a Syringe Infusion Pump to deliver consistent flow rates and dependable alarms under demanding conditions.
Tender specifications reflect these expectations with clear technical benchmarks and compliance mandates.
Understanding Hospital Tender Priorities
Hospitals prioritise safety, reliability, regulatory compliance, and lifecycle value in tender documentation.
A Syringe Infusion Pump must meet defined accuracy tolerances, alarm parameters, and usability standards.
Procurement teams carefully assess whether devices align with institutional protocols and national regulations.
Tender committees often include clinicians, biomedical engineers, and finance officers.
Each stakeholder evaluates the Syringe Infusion Pump from a different perspective, yet all focus on patient safety.
Alignment requires balancing clinical functionality with operational and financial sustainability.
Key hospital tender priorities generally include:
-
Flow rate accuracy within defined tolerance ranges
-
Clear alarm systems with occlusion detection and safety alerts
-
Battery backup suitable for transport and power interruptions
-
Compatibility with multiple syringe brands and sizes
-
Robust build quality for high-dependency environments
-
Compliance with national and international regulatory standards
These specifications ensure that the Syringe Infusion Pump supports uninterrupted and safe medication delivery.
Hospitals rely on documented testing results and certification evidence to verify compliance.
Manufacturers who provide transparent documentation build trust during the evaluation stage.
Clinical Accuracy and Safety Features
Accuracy remains the cornerstone of any Syringe Infusion Pump tender requirement.
Hospitals define acceptable infusion rate deviations to minimise medication errors.
Precise calibration and validated performance testing are therefore essential components of compliance.
Safety features such as occlusion detection and anti-bolus protection are equally critical.
Tender documents frequently require multi-level alarm systems with both visual and audible indicators.
A Syringe Infusion Pump must demonstrate consistent response times during simulated clinical scenarios.
Clear display interfaces also influence clinical acceptance.
Nurses working in high-pressure ICU settings require intuitive controls and readable screens.
Human-centred design ensures that the Syringe Infusion Pump supports workflow efficiency rather than complicating it.
Regulatory Compliance and Quality Assurance
Hospital tenders emphasise regulatory alignment to safeguard institutional reputation.
Compliance with recognised standards confirms that the Syringe Infusion Pump has undergone rigorous validation.
Certification documents form an integral part of technical bid submissions.
Quality assurance processes must extend beyond production to post-market monitoring.
Hospitals expect manufacturers to maintain traceability and structured complaint handling systems.
A dependable Syringe Infusion Pump supplier demonstrates commitment to continuous improvement.
Important compliance considerations often include:
-
ISO certification for quality management systems
-
Electrical safety and electromagnetic compatibility standards
-
Clinical evaluation reports and risk management documentation
-
Manufacturing process validation records
-
Post-market surveillance frameworks
Such documentation reinforces the credibility of the Syringe Infusion Pump within competitive tenders.
It also reassures hospitals that long-term reliability has been thoroughly assessed.
Regulatory transparency strengthens institutional confidence in device adoption.
Operational Compatibility and Integration
Modern hospitals operate interconnected digital ecosystems.
A Syringe Infusion Pump must integrate seamlessly with hospital information systems where required.
Tender requirements may therefore include connectivity or data logging capabilities.
Interoperability supports medication documentation and clinical audits.
Hospitals benefit from devices that contribute to streamlined record-keeping and performance review.
A Syringe Infusion Pump that aligns with digital workflows enhances operational visibility.
Physical compatibility is equally important.
Compact design, stackable configurations, and pole-mount flexibility assist in optimising ICU space.
Hospitals often specify environmental durability to withstand continuous clinical use.
Lifecycle Value and Cost Considerations
Tender evaluation extends beyond initial purchase price.
Hospitals assess the total cost of ownership across the device lifecycle.
A Syringe Infusion Pump should demonstrate durability, service support, and accessible spare parts.
Preventive maintenance requirements influence operational planning.
Biomedical teams prefer devices with straightforward calibration and diagnostic procedures.
Ease of servicing reduces downtime and protects patient care continuity.
Lifecycle evaluation may consider:
-
Warranty duration and service response time
-
Availability of local technical support
-
Spare parts accessibility and pricing transparency
-
Energy efficiency and battery longevity
-
Training programmes for clinical and technical staff
These factors ensure that the Syringe Infusion Pump remains dependable throughout years of clinical service.
Hospitals value partnerships that prioritise sustainability and long-term reliability.
Transparent service commitments strengthen tender competitiveness.
Training and Clinical Support
Effective deployment requires structured training programmes.
Hospitals expect manufacturers to provide comprehensive user education during installation.
A Syringe Infusion Pump should be accompanied by practical demonstrations and competency validation.
Clinical support enhances safe adoption.
Hospitals benefit when suppliers offer guidance on protocol alignment and safe operating procedures.
Training documentation often forms part of tender requirements.
Clear manuals, multilingual instructions, and ongoing support contribute to consistent device usage.
A well-supported Syringe Infusion Pump minimises misuse and enhances caregiver confidence.
Education therefore becomes an essential element of tender alignment.
Evaluating the Right Manufacturing Partner
Beyond product specifications, hospitals evaluate the credibility of the manufacturer.
Experience in critical care equipment reflects understanding of complex clinical environments.
A reliable Syringe Infusion Pump manufacturer demonstrates stable production capacity and ethical practices.
Manufacturing infrastructure, quality audits, and supply chain transparency matter deeply.
Hospitals prefer partners who invest in research and continuous technological advancement.
Alignment with institutional values fosters long-term collaboration.
Reputation, documented performance history, and responsible service networks influence final selection.
Hospitals seek assurance that their Syringe Infusion Pump supplier will remain dependable over time.
Strong partnerships ultimately support improved patient outcomes.
Conclusion
Aligning Syringe Infusion Pump features with hospital tender requirements demands more than technical compliance.
It requires understanding the clinical, operational, and ethical responsibilities embedded within procurement processes.
Hospitals depend on reliable, accurate, and safe drug delivery systems to protect every patient entrusted to their care.
When manufacturers prioritise regulatory transparency, safety validation, lifecycle value, and clinical training, tender alignment becomes meaningful rather than procedural.
Thoughtful design and responsible quality assurance ensure that each Syringe Infusion Pump supports healthcare professionals in delivering precise treatment.
Such alignment builds confidence, strengthens institutional trust, and promotes sustainable healthcare delivery.
Manufacturers committed to innovation and safety continue to shape the future of infusion therapy.
Akasinfusion manufactures world-class Drug Delivery devices such as volumetric pumps, supporting hospitals with dependable technology built around patient wellbeing.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Oyunlar
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness



